DATAMYTE additionally enables you to conduct layered process audits, a high-frequency evaluation of critical process steps, focusing on areas with the highest failure risk or non-compliance. Conducting LPA with DATAMYTE lets you effectively identify and correct potential defects earlier than they turn out to be main quality points. With Quality 4.zero, quality is now not about catching defects after the fact—it’s about stopping them from happening within the first place.
Here, quality is at all times relative to a consumer—their wants, sources, and safety. In Juran’s definition, the standard of one thing is dependent upon how someone will use it. It’s not nearly making sure your merchandise are perfect—it’s additionally about making sure they’re the best they can be in order that your clients shall be happy and hold coming again for more. Once we perceive that Quality is the absence of waste, we are able to see that there are two associated approaches that we will employ to eliminate waste and, thus, improve the Quality of our methods. According to LNS Research, there could be widespread recognition of AI’s potential to enhance information-gathering duties in high quality processes.
From there, an enterprise QMS could provide an inventory of other solutions, either packaged collectively or bought individually. Examples include criticism handling, deviation and non-conformance administration, supplier high quality management and more. A QMS is an important device for companies pursuing course of excellence using frameworks such as Lean, Six Sigma or Total Quality Management (TQM).
Q6: How Do Environmental Components Affect Manufacturing Quality?
Documenting processes, gathering information, tracking results—all of these are simplified with an automated QMS, in order that groups have more time to concentrate on strategy somewhat than administrative tasks. A sturdy QMS reduces the probability of external audit and buyer audit findings. Standardizing your processes and maintaining proper documentation helps reveal compliance and give prospects according to the manufacturing-based definition of quality: and external auditors confidence in your operations. A QMS is a formal system with documented processes, procedures and obligations for achieving quality objectives and insurance policies. The key word right here is “documented,” underscoring the importance of documentation and recordkeeping in high quality. It should be no shock that the ASQ suggests producers add agile methodology to their lean programs.

This, in turn, can have a positive downstream effect on metrics such as scrap, overall tools effectiveness (OEE) and cost of quality. Consistently assembly or exceeding quality necessities and targets is the necessary thing to making sure customer satisfaction—the driving aim of high quality. Quality assurance is a process that includes planning, designing, and implementing methods and processes to ensure that products or services meet specified requirements.
Across all accepted definitions, quality is relative to a shopper, a product, and an consequence. At the same time, manufacturers do their best to scope the necessities of the buyer. A product that conforms to technical specifications however fails to meet the consumer’s want isn’t conforming to necessities. As a finest follow, producers will try to perceive and eliminate the source of nonconformances whenever they arise.
Built-in Level Solutions Vs Enterprise Qms?
For instance, whereas all the components that the customer obtained met the spec, what if, so as to do so, we had to pay a crew a half shift of additional time to inspect? The further cost, whereas not passed directly to the customer, nonetheless inflates our cost of doing business and, thus, makes our choices more expensive. The buyer, as a outcome of they bid out jobs, understands implicitly that our offering is inferior — as a outcome of they can examine the terms and prices that others offer. With a QMS in place, you’ll be able to ensure that you are addressing and resolving quality points shortly. With QMS software program, manufacturers can centralize quality information in one place.

For many manufacturing firms, deploying point solutions individually is a less expensive possibility that enables them to target specific processes with advanced performance. ISO has published steerage on what it considers the seven core high quality administration rules, which producers can use as a reference when implementing a QMS. Left unchecked, these challenges can result in non-conformances, defects or deviations. If these problems usually are not caught earlier than delivery, you’ll be dealing with customer complaints, expensive product recalls and rework. Companies without an effective QMS also put themselves vulnerable to expensive regulatory violations and penalties. “Quality is Free” is the title of a book written by quality consultant, Philip Crosby in 1979.
This allows you to observe tendencies and patterns over time, identify areas for enchancment and mitigate the chance of high quality escapes. All of this will result in a broken reputation and loss of key enterprise contracts. Implementing a robust QMS empowers you to determine quality issues early and repeatedly optimize your processes. Companies can use data to spot patterns and take corrective action earlier than issues snowball out of control, freeing up resources for other strategic priorities like product innovation. Quality four.0 is all about using data and technology to enhance high quality in manufacturing.
While manufacturing organizations usually are not required by law to observe this commonplace, in plenty of instances OEM prospects require their suppliers to be ISO 9001-certified. Through the standardization of processes and documenting procedures, a QMS reduces errors, minimizes waste and optimizes assets. An automated QMS makes it easier to identify and remove bottlenecks in your process, resulting in improved productiveness and cost financial savings. A systematic method to implementing a QMS helps manufacturers meet buyer and regulatory requirements while continuously enhancing its effectiveness and effectivity. It additionally ensures that everybody in the group understands their function in attaining high quality aims.
‘quality As The Absence Of Waste’
Still, high quality returns to designing and producing goods that work for the tip user. The beauty of this definition is that it applies to all manufactured merchandise, from probably the most advanced prescribed drugs to automobile parts. It applies equally well to industries where any new product requires months of regulatory approval and testing and industries the place new merchandise can roll off the lines at a moment’s discover. Still, there are commonalities that unite definitions across trade and product. Quality is the absence of waste in course of and in our human performers.
- Conducting LPA with DATAMYTE enables you to successfully establish and proper potential defects before they turn into major quality points.
- Quality requirements for PCBs aren’t essentially relevant for a meals and beverage producer.
- Features of high quality for, say, cutting-edge biologics, will differ from those for vehicle components.
- Quality management procedures are essential for catching errors and guaranteeing that merchandise meet or exceed buyer expectations.
- At the top of the day, quality in manufacturing is important as a result of it’s what prospects expect.
Conversely, a single negative expertise as a outcome of poor high quality can damage your model image and customer loyalty. The preliminary outlay is usually larger, and implementation is normally a prolonged course of. If functionality in a given solution doesn’t align with your process, you could be left making an attempt to shoehorn your course of to suit the software program. The downside is that this can erase any effectivity positive aspects you’ve remodeled time fine-tuning your high quality processes, making flexibility essential.
Benefits Of A Quality Administration System In Manufacturing
There are many various quality management procedures, so you’ll need to choose on the ones which might be right for your business. Customer feedback is significant because it provides insights into real-world product performance and customer satisfaction. This information can result in enhancements in product design, performance, and quality control processes, enhancing total product high quality. A QMS helps scale back defects, reduce customer complaints and enhance on-time delivery.

Your employees play a big role in figuring out the standard of your products. If you rent certified, skilled staff, they will be extra prone to create high-quality products. On the opposite hand, should you hire unqualified or inexperienced employees, they will be more prone to create products of poor quality. Your buyer tells you once they look for different suppliers, that there’s waste in your choices. Continuous enchancment of the individuals and processes in your authority is the step you must take to enhance your company’s Quality.
What they discover is that whereas overall defects have decreased by 20%, process data reveals there are still instances where operators aren’t following the brand new course of. Point answer software distributors typically target only one process, aiming for deep functionality in that space. With the instruments of Quality four.zero at their disposal, producers have a chance to serve their clients better than ever before. For one, new applied sciences have raised the ceiling when it comes to repeatability, efficiency, and consistency in high quality.
High Quality Standards And Rules In Manufacturing
The Plan-Do-Check-Act (PDCA) cycle, popularized by William Deming and based on the sooner Shewhart Cycle, is a cornerstone of continuous enchancment in high quality administration. Both options provide manufacturers a quantity of benefits, together with improved efficiency, regulatory compliance, knowledge administration and real-time monitoring of quality issues. At the highest of the requirements record is ISO 9001, which is probably the most recognized high quality administration standard on the earth.
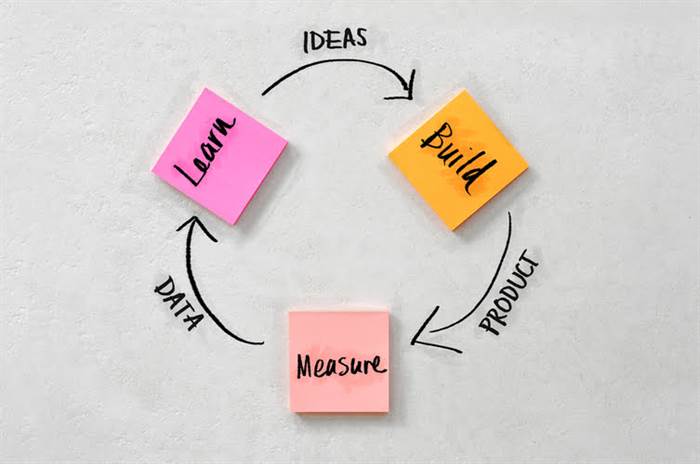
In the previous, quality was mostly about catching defects after the precise fact and fixing them. But with Quality 4.0 and the data-driven method it allows, we can now prevent defects from occurring within the first place. This is a huge shift that’s making manufacturing extra environment friendly and effective.
Contact us right now to study more about how DATAMYTE may help you enhance the quality of your products and exceed customer expectations. We are devoted to helping businesses obtain Quality 4.zero and drive continuous improvement, making manufacturing extra environment friendly and effective than ever earlier than. There are many definitions of quality in manufacturing, however an important thing to remember is that quality meets or exceeds buyer expectations. This means you should produce merchandise that meet or exceed your customers’ wants and needs. Quality additionally contains options like durability, reliability, and aesthetic enchantment. Quality management procedures are essential for catching errors and making certain that merchandise meet or exceed buyer expectations.
We’ll also examine the ongoing transition in manufacturing from paper-based quality techniques to focused digital options, together with tips on how to implement a QMS that drives continuous improvement. Second, the shift toward agile manufacturing has brought new consideration to end-to-end product development. As all of those definitions of high quality argue, quality starts with product design and continues by way of use by the consumer. New technologies have spurred a resurgence of interest in quality in manufacturing–enough to earn the name Quality 4.zero. Given this return to high quality, now is an effective time to revisit a few of the canonical definitions of high quality. They still have so much to teach us about high quality initiatives within the present, and where they could go sooner or later.


